State Key Laboratory of Medicinal Chemical Biology
Photodynamic
therapy holds great potentials in cancer treatment, yet its effectiveness in
hypoxic solid tumor is limited by the oxygen-dependence and insufficient
oxidative potential of conventional type II reactive oxygen species (ROS).
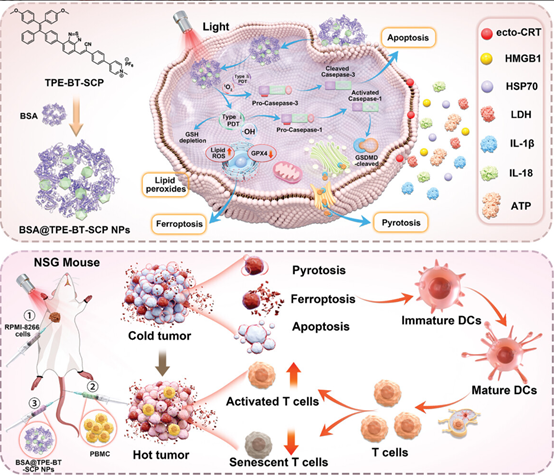
Herein, the study reports a supramolecular photosensitizer, BSA@TPE-BT-SCT NPs,
through encapsulating aggregation-enhanced emission photosensitizer by bovine
serum albumin (BSA) to significantly enhance ROS, particularly less
oxygen-dependent type I ROS for photodynamic immunotherapy. The abundant type I
ROS generated by BSA@TPE-BT-SCT NPs induce multiple forms of programmed cell
death, including apoptosis, pyroptosis, and ferroptosis. These multifaceted
cell deaths synergistically facilitate the release of damage-associated
molecular patterns and antitumor cytokines, thereby provoking robust antitumor
immunity. Both in vitro and in vivo experiments confirmed that BSA@TPE-BT-SCT
NPs elicited the immunogenic cell death, enhance dendritic cell maturation,
activate T cell, and reduce myeloid-derived suppressor cells, leading to the
inhibition of both primary and distant tumors. Additionally, BSA@TPE-BT-SCP NPs
also exhibited excellent antitumor performance in a humanized mice model,
evidenced by a reduction in senescent T cells among these activated T cells.
The findings advance the development of robust type I photosensitizers and
unveil the important role of type I ROS in enhancing multifaceted tumor cell
deaths and antitumor immunogenicity.
 津公网安备 12010402001780号
津公网安备 12010402001780号








